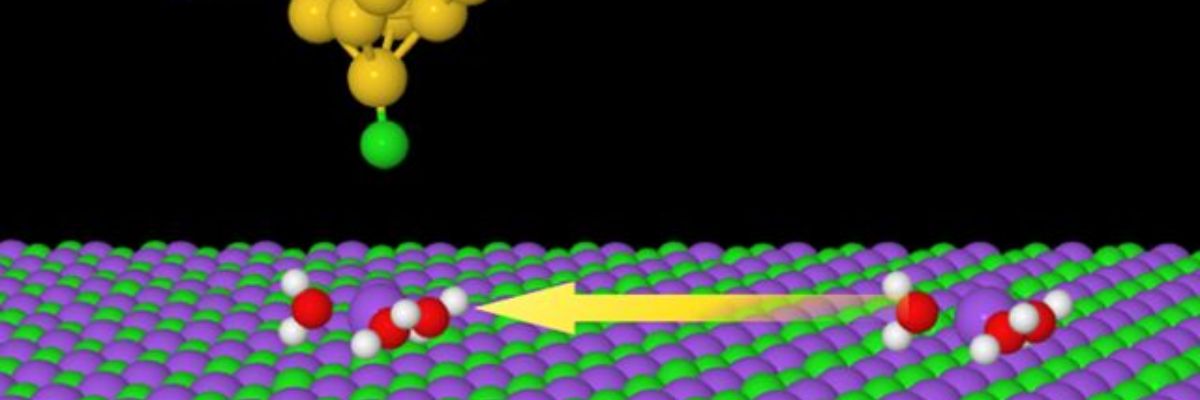
Scientists have managed to show the movement and structure of ionic hydrates
17. 05. 2018
Without ionic hydrates of sodium, cells could not function, dissolving with salt. We find them in ionic drinks. The hydration of ions has its role in corrosion even in electrochemistry. “We have managed to fill one of the blank places in chemistry, biology and physics,” said Pavel Jelínek from the Institute of Physics of the CAS. The unique study by Czech and Chinese scientists has been published in the journal Nature in recent days.
“We have taken another step towards understanding how the transport of hydrates works and what influence their structure has on their mobility. Nobody else has yet been able to study these substances, their movement and structure with such precision,” stated Pavel Jelínek, who also works at Palacký University in Olomouc.
Eyes to the nanoworld
The possibility to see the individual molecules, manipulate them and even depict the chemical bonds was still unimaginable relatively recently. It was only made possible by the development of scanning microscopes with atomic resolution, which has opened new possibilities to scientists for knowledge inter alia in the area of the characterization and modification of nanostructures.
Our Chinese colleagues first prepared various types of clusters, when various numbers of water molecules attached to atoms of sodium. Scientists subsequently investigated the results of these atomic games using specially modified scanning microscopes, but the fundamental discovery was not possible without special methods, on the development of which Czech physicists participated significantly and in January this year published in it in the journal Nature Communications.
The mobility of the ions influence corrosion
“The problem in the study of these clusters are their relatively weak internal bonds, which the tip of the microscope easily disturbs. With the aid of a new imaging method we can overcome this obstacle by hanging a molecule of carbon monoxide on the tip. Thanks to its presence, we are able to see not only whether the ion is surrounded by one, two or perhaps three water molecules, but we observe also the arrangement of the individual molecules, without having disrupted the structure of the studied cluster of ionic hydrate,” explained Jelínek.
When scientists knew the composition and appearance of the studied clusters in detail, using an electric pulse they moved them and measured their mobility. It is generally true that the greater the mobility of the cluster, the more effective they are. “We discovered that the ion of sodium hydrated with three water molecules moves the fastest. It arises from that that precisely this type of cluster will play a significant role for the possibility of the management of the transport of sodium in biology or to influence corrosion,” added Jelínek.
Prepared by Vlaďka Coufalová, Department of Media Communication of the Head Office of the CAS in cooperation with the Institute of Physics of the CAS
Photo: Archive of the Institute of Physics of the CAS
Read also
- Three new lakes under the ice in Antarctica
- The missing information on photosynthesis is revealed by lasers
- Putovní výstava „Umění vědy“ v Jihlavě
- Letní škola astronomie v Brně
- Poprvé na světě: Protony urychlené v plazmatu vytvářeném z vodíkového ledu laserem PALS
- Praha zažije největší setkání sociologů a socioložek v evropské historii
- Finále Expedice vesmír 2015
- Nejpřesnější test základního zákona mikrosvěta – symetrie CPT
- V obecném zájmu
- Vědecká spolupráce s Korejskou republikou
The Czech Academy of Sciences (the CAS)
The mission of the CAS
The primary mission of the CAS is to conduct research in a broad spectrum of natural, technical and social sciences as well as humanities. This research aims to advance progress of scientific knowledge at the international level, considering, however, the specific needs of the Czech society and the national culture.
President of the CAS
Prof. Eva Zažímalová has started her second term of office in May 2021. She is a respected scientist, and a Professor of Plant Anatomy and Physiology.
She is also a part of GCSA of the EU.